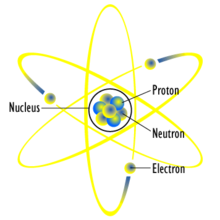
Figure 1: the atom
For you people who do not know what the atomic structure of an atom is, or if you need a refresher course in atoms, here are the basic parts of an atom. The atom is made up of three basic particles; protons, neutrons and electrons. Protons are positively charged particles and neutrons have a neutral charge. These two particles can be found the atom's nucleus.
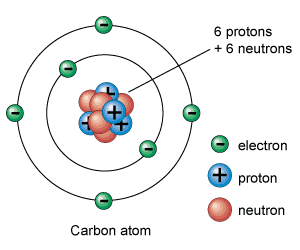
Figure two: the atom
The electrons spin around the atom's nucleus in a random and spherical orbit, and this forms an electron cloud. The next most important thing you need to know about is shells. No, not like egg shells but electron shells. Electron shells are the fixed position that certain electrons travel on. Atoms can have one, two or even seven electron shells. In each electron shell, a certain amount of electrons can be held in them. The first shell can hold up to two electrons, the second and third one can hold up to eight, the fourth and fifth can hold up to nine electrons each, and the sixth and seventh can hold up to thirty-two each. You should also know what valance electrons are; valence electrons are the electrons that atoms share or take away from one another, and are located on the atoms outermost shell. With this information, let's learn about chemical bonds.
Chemical bonds are the attraction of atoms when an atom shares, takes away or loses electrons. Atoms bond to become stable. A stable atom is one that has a full outer shell of electrons versus an unstable atom which does not have a full outer shell. The two most common bonds you will hear about are ionic and covalent bonds. A covalent bond is formed when two or more atoms share electrons to achieve a full outer shell. An example of this is H20. An ionic bond is formed when an atom takes away another atoms electron. When this happens, the atom that took away the electron becomes either positively or negatively charged and the other atom to lose the electron changes charge too, but due to electrostatic attraction opposites attract and the two atoms bond. An example of this is salt.
figure three: a covalent bonds
Thanks for reading the blog, remember leave a comment in the comment section down below.