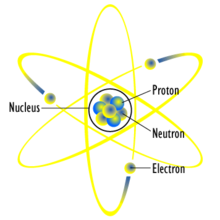
Atoms are the smallest part of an element that can still retain its chemical properties. The Atom is made up of protons, neutrons, and electrons. The protons are positively charged, neutrons are a neutral charge, and of course electrons are negatively charged. The neutrons and protons can be found in the nucleus being held together by the strong force. The strong force keeps the protons from repelling each other. Outside of the nucleus, spinning at almost the speed of light, is the negatively charged electrons.
Figure 1: Diagram of an atom
When two or more different types of atoms bond together, they form a molecule. A great example of a molecule is water. Water, also known as H2O, is formed by two hydrogen and one oxygen atoms coming together. It is believed that the first molecules formed about 300,000 years after the Big Bang, or just under 15 billion years ago.
Figure 2: A water molecule
About 2,400 years ago, a Greek philosopher named Democritus
came up with the brilliant idea that the world was made up of tiny particles
that could not be cut in half. He called these particles atoms from the Greek word atomos, meaning indivisible. Later on in the 1800s a man named John Dalton refined
the idea of the atomic theory. He claimed that an atom is the smallest part of
an element that can still retain its chemical properties. A simpler way to think
about it is that if you were to break an element like iron to its atomic size, it
would still be iron, but if you try and break the iron atom any smaller, it
would not be iron anymore. Though many of his colleagues were not fond of his
theory, it eventually became accepted through many more
experiments conducted by various people.

Figure 3: Democritus
Figure 4: John Dalton
Hoped you liked the blog, please leave a comment in the comment section down below.
Additional Links
www.johndalton.org
education.jlab.org/atomtour
http://www.bing.com/videos/search?q=Bill+Nye+Atoms+And+Molecules&form=HDRSC3&first=1#view=detail&mid=A43F6273E7F9E678CDBEA43F6273E7F9E678CDBE
http://www.youtube.com/watch?feature=player_embedded&v=cnXV7Ph3WPk

No comments:
Post a Comment